Question 1
a) Differentiate between a compound and a mixture
b) Give one example of a miscible liquid-liquid mixture.
Answer
a) A Compound is a pure substance made up of two or more elements chemically combined while a Mixture consists of two or more substances in which components making up the mixture retain their physical and chemical properties/ Consists of two or more substances which can be separated by chemical means.
b) Water and ethanol
b) Water and ethanol
Question 2
a) Draw a diagram of a volumetric flask.
b) Explain how it is adapted to measure accurate volume.
Answer
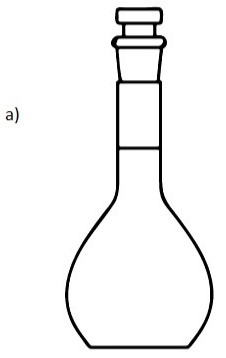
b) It has narrow neck to reduce the error that results from meniscus.
Question 3
The diagram below shows a type of flame produced by a Bunsen burner
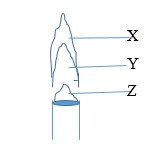
a) Give the name to the flame above
b) Name the parts labelled X, Y and Z.
c) Which part is the hottest? Explain.
Answer
a) Non luminous flame
b) X- Pale blue zone
Y- Green blue zone
Z- Almost colourless zone
c) X Pale blue zone. There is complete combustion of the laboratory gas due to enough air.
b) X- Pale blue zone
Y- Green blue zone
Z- Almost colourless zone
c) X Pale blue zone. There is complete combustion of the laboratory gas due to enough air.
Question 4
Describe how a mixture of solid potassium chloride and iodine can be separated.
Answer
- Put the mixture in a glass beaker covered with watch glass containing ice cold water.
- Heat the mixture. Iodine sublimes and deposits on the cooler parts.
- Heat the mixture. Iodine sublimes and deposits on the cooler parts.
Question 5
Write the equations for the following reactions.
a) Burning carbon in excess air.
b) Heating solid iodine and cooling.
Answer
a) Carbon + Oxygen ⟶ Carbon (IV) oxide
b) Solid iodine ⇌ Iodine vapour
b) Solid iodine ⇌ Iodine vapour
