Question 1
The grid below forms part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
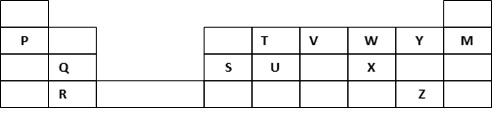
- Write the general name given to the element P belong.
- An element N has an atomic number of 15. Write down its electronic arrangement and hence fix it in its right position on the grid above.
Electronic arrangement - Compare the size of the atom of R and that of its ion. Explain your answer.
- Give the formula of the compound formed between
i. P and W
ii. T and Y - Compare the melting points of element Q and S. Explain
- State the least reactive element in the grid. Give a reason for your answer
- Give two advantages that element S has over element Q in making electric cables
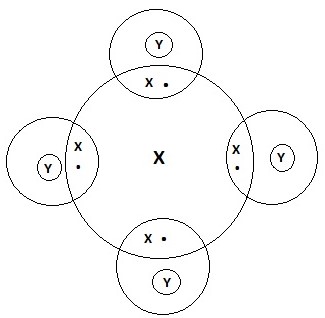
- Draw a dot (.) and cross (x) diagram to represent the bonding in compound formed between T and Y
Answer
- Alkali metals
- Electronic arrangement - 2.8.5
- R has a bigger atomic size than its' ions because R looses electrons hence an energy level lost therefore making the size of its ion to be smaller than the atom.
- i. P and W - P2W
ii. T and Y - TY4 - S has a higher melting point than Q. This is because S has a stronger metallic bond than Q (three electrons in outermost energy requiring alot of heat to break the bond in S than Q.
- M. It is stable, does not need to react with other elements to gain stability.
- 1. It is ductile
2. It does not corrode -
Question 2
Study the table below and answer the questions that follow
| Bond type | Bond energy Kjmol-1 |
|---|---|
| C-C | 346 |
| C=C | 610 |
| C-H | 413 |
| C-Br | 280 |
| Br-Br | 193 |
- Calculate the enthalpy change for the following reaction
C2H4(g) + Br2(g) → C2H4Br2(g) - Name the type of reaction that took place in above
Answer
- Bond breaking
C=C-1
C-H=4
Br-Br-1
610 + 4(413) + 193
= 2455
Bond formation
C-C = 1 x 346
C-Br- 2 x 280
C-H = 4 x 413
= 2558
∆H = 2455 - 2558
= - 103Kjmol- - Addition reaction
Question 3
Butane C4H10 cannot be prepared directly from its elements but its standard heat of formation (∆) can be obtained indirectly.
The following heats of combustion are given
∆ (Carbon) = -393kJ/mol
∆ (Hydrogen) = -286kJ/mol
∆ (Butane) = -2877kJ/mol
- Draw an energy cycle diagram linking the heat of formation of butane with its heat of combustion and the heat of combustion of its constituents elements.
- Calculate the heat of formation of butane ∆ (C4H10)
Answer
-
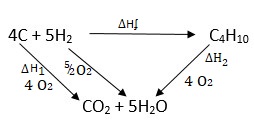
- ∆H1 = ∆Hʄ + ∆H2
∆Hʄ = ∆H1 - ∆H2
4( -393) + 5(-286) - (-2877)
-3002 + 2877
= -125Kjmol-1
Question 4
Given that the lattice enthalpy of potassium chloride is +690kJ/mol and hydration enthalpies of K+ and Cl- are -322kJ and -364kJ respectively. Calculate the enthalpy of solution of potassium chloride.
Answer
∆Hs = ∆Hlatt + ∆Hhyd
= 690 + 322 + (-364)
= 690 - 686
= +4 Kjmol-1
= 690 + 322 + (-364)
= 690 - 686
= +4 Kjmol-1
Question 5
The diagram below represents the Haber process for the manufacture of ammonia. Study it and answer the questions that follow.
-6n14p3zT2qAAqqlN.jpg)
- Name any two impurities removed by the purifier.
- The catalyst used in the process is finely divided iron. Why iron is finely divided?
- In the Haber process the conversion of nitrogen and hydrogen into ammonia is only 10%. The remaining unreacted gases are recycled. What is the advantage of this?
- A part from iron catalyst and pressure of 500 atmospheres, name any other condition required for this process.
Answer
- 1. Carbon (iv) Oxide
2. Sulphur (iv) Oxide - To increase its surface area.
- Reduces wastage.
- Temperature of 450 - 500oC
