Question 1
The diagram below shows the apparatus for the preparation of gas A and investigate on its properties . Study it and answer the questions that follow.
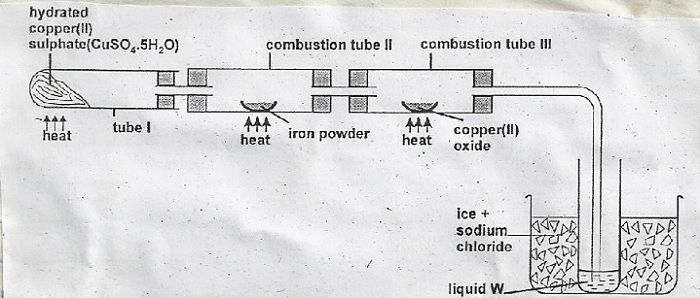
- (i) Name gas A
(ii) Suggest the property of gas A under investigation.
(iii) Write chemical equations for the reactions in the;
Boiling tube I
Combustion tube II - (i) State and explain the observation made in
Tube I
Combustion tube II - (i) What is the use of hydrated copper (II) sulphate in the experiment.
(ii) Name one other substance that comes out of tube III.
(iii) Name liquid W
(iv) What is the role of sodium chloride in the ice (freezing mixture).
(v) Explain why hydrogen gas has been replaced by helium in filling of aeroplane tyres.
Answer
- (i) A - Hydrogen
(ii) Reducing agent
(iii) tube I - CUSO45H2O → CUSO4(s) + 5H2O
tube II - 3Fe(s) + 4H2O(g) → Fe3O4 + 4H2(g) - (i)
Tube I - Blue solid turns white/colourless liquid; loss of water of crystallization.
Combustion tube II - Black solid turns brown. copper (ii) oxide reduced to copper metal. - (i) To produce steam
(ii) hydrogen
(iii) W - water
(iv) Decrease the freezing point of water
(v) Hydrogen is flammable
Question 2
The flow diagram below shows the reactions involved in the process for the preparation and reactions of salt C. Study it and answer the questions that follow.
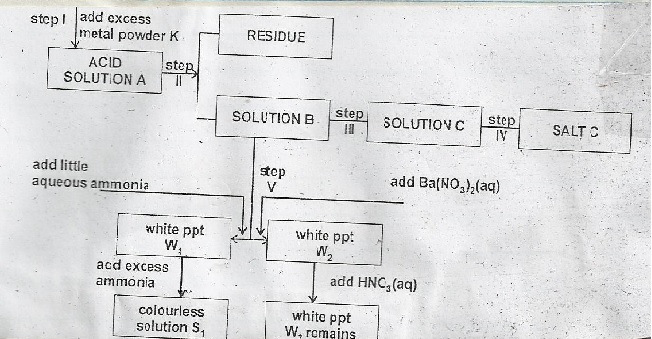
- Identify
(i) Metal K
(ii) Acid A
(iii) Salt C - In step III the solution B is transferred into an evaporation dish and heated in a water bath until it is saturated.
(i) What is a saturated solution?
(ii) Why is heating done over a water bath?
(iii) How would one determine whether a solution is saturated? - Explain why metal powder K is used in excess
- Name step (II) and state its importance.
- Identify
(i) White precipitate W1
(ii) White precipitate W2
(iii) Colourless solution S1 - Write equations for step I and for the formation of S1
Equation step I
Formation of S1
Answer
-
(i) Metal K - zinc
(ii) Acid A - Dilute sulphuric (vi) acid
(iii) Salt C - zinc sulphate -
(i) A solution that cannot dissolve any more of solute at given temperature.
(ii) Allow crystallization
(iii) Dip a glass rod when heating. Allow solution on glass rod to cool. Formation of crystal. - To ensure all the acid have reacted.
- Filtration: To remove excess metal K.
-
(i) W1 - Zinc hydroxide
(ii) W2 - Barium sulphate
(iii) S1 - Tetra ammine zinc (ii) hydroxide -
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H(g)
Zn(OH)2(s) + 4NH3(aq) → (Zn(NH3)4(aq))2+ + 2OH-(aq)
Question 3
- Sulphur is allotropic. What does this mean?
- Give two differences between rhombic and monoclinic sulphur.
- State and explain using an equation the observations made when sulphur reacts with hot concentrated nitric (v) acid.
Observations
Equation
Answer
- It can exist in several forms without change of state.
-
Rhombic Monoclini Melting point 1140 C Melting point 1190 C Stable below 960 C Stable above 960 C Octahedral Needle like -
Observations: - Brown fumes, pale yellow liquid or colourless liquid.
Equation: S(s) + 6HNO3 → H2SO4(aq) + 6NO2(g) + 2H2O(l)
Question 4
The flow chart below shows how sulphuric acid is manufactured in a large scale. Study it and then answer the questions that follow.
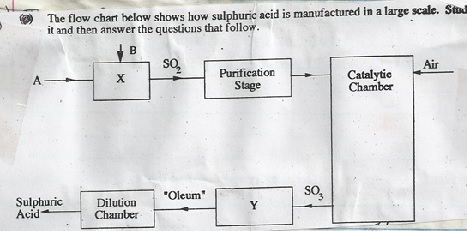
- (i) Name the raw materials A and B
A
B
(ii) Name the chambers X and Y.
- (i) Name two impurities that are removed during the purification stage.
(ii) Why must the impurities in (i) above be removed. - (i) Name the catalyst used in this process.
(ii) The equation below shows what happens in the catalytic chamber.
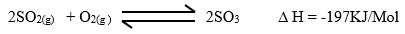
State the two conditions that are necessary for maximum production of SO3.
Answer
- (i)
A - Sulphur
B - Oxygen
(ii) X - burner/Roaster
Y - Absorption tower
- (i) - Dust particles
- Carbon (iv) oxide
(ii) To avoid poisoning of the catalyst. - (i) Platinum/vanadium (v) oxide.
(ii) - High pressure
- Low temperature of 400 – 500oC
Question 5
Define the term standard heat of formation of a substance.
Answer
This is heat absorbed / evolved / heat change when one mole of any substance is formed from its constituent elements.
