Question 1
Two elements X and Y have their atomic number being 11and 19 respectively.
- Show their electronic configurations
- Are the elements in the same period or group ,give a reason for your answer.
- Compare the reactivity of the two elements.
Answer
- X - 2.8.1
Y - 2.8.8.1 - Same group, because they both have one electron in the outermost energy level.
- Y is more reactive than X.
Question 2
State the type of bond in each of the following compounds.
- Calcium fluoride
- Methane
- Ammonium ion
Answer
- Calcium fluoride - Ionic bond
- Methane - Covalent bond
- Ammonium ion - Covalent and dative bond
Question 3
Explain why the melting point of sodium is higher than that of potassium.
Answer
Small atomic radius hence stronger metallic bonds.
Question 4
State the uses of the following apparatus in chemistry.
- A separating funnel
- A pipette
- A spatula
Answer
- A separating funnel - Separating immiscible liquids.
- A pipette - Measuring small accurate volumes.
- A spatula - Scoping small amounts of solids.
Question 5
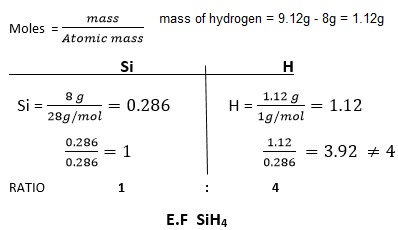
9.12g of a gaseous compound contains 8g of silicon while the rest is hydrogen. Determine the empirical formula of the compound. (H = 1, Si = 28).
Answer