Question 1
The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
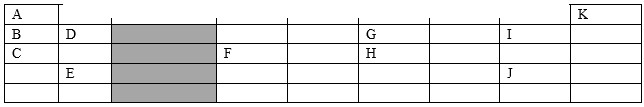
- Which letter represents an element that is least reactive
- Why are elements D and E referred to as alkaline earth metals
- How does the atomic radius of F and H compare?
- Select two letters representing a pair of elements that would react most explosively.
- Write an equation showing how element D forms its ions
- Write the formulae of :
- Bromide of D
- Sulphate of C
- What type of bonding exist between
- E and J
- G and J
- Explain why the melting point of J is higher than that of I
- When 1.15g of C were reacted with water, 600cm3 of gas was produced. Determine the reactive atomic mass of C. (Molar gas volume = 24 000cm3)
Answer
- K
- Because elements D and E are found in group II of the periodic table are found widely in the earth's crust.
- F has a larger atomic radius than H because across the periodic there is increase of effective nuclear charge due to increase in the number of protons.
- Elements C and I
- D(g) → D2+(g) + 2e-
-
- Bromide of D - DBr2
- Sulphate of C - C2SO4
- E and J - Ionic bond
- G and J - Covalent bond
- The M.P of J is higher than that of I because as you move down the group the molecular mass increases with the size hence the bigger the molecule the higher the number of Van der wall forces.
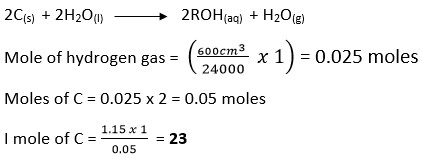
Question 2
The flow chart below outlines the manufacture of several important fertilizer. Use it to answer the questions that follow:
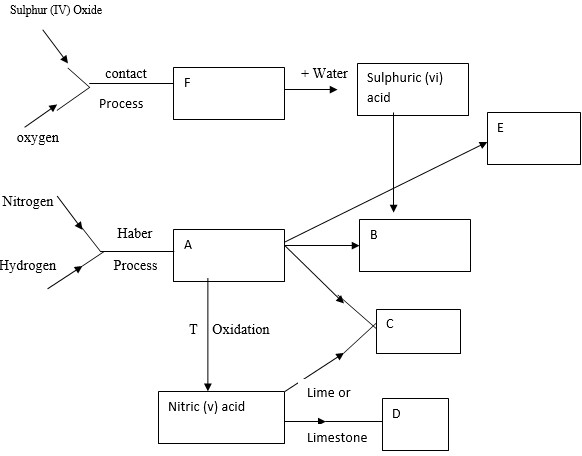
- Identify substance A,B,C,D,E and F
- A
- B
- C
- D
- E
- F
- Identify substance A,B,C,D,E and F
- From which industrial process can sulphur be obtained?
- State the condition necessary for conversion of sulphur (IV) oxide to sulphur (VI) oxide in the contact process.
- State one source of hydrogen used in the haber process.
-
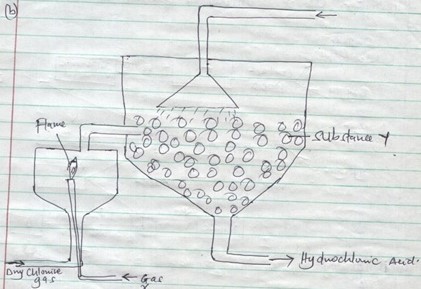
- Name substances X and Y
- Give one source of substance X used in the process above
- What is the purpose of substance Y in the process above?
- Write an equation from the reaction producing hydrogen chloride gas in the above process.
Answer
-
- A - Ammonia
- B - Ammonium sulphate
- C - Ammonium Nitrare
- D - Calcium nitrare
- E - Nitric (v) oxide
- F - Sulfur trioxide
-
- Sulfur can be obtained as a byproduct in the copper refining process or through the distillation of natural gas.
- Presence of a catalyst(Vanadium (v) Oxide)
Temperature: Approximately 450°C to 500°C
About 1 to 2 atmospheres
- Natural gas, which is primarily Methane.
-
- X - Hydrogen gas
Y - Glass beads - Hydrogen gas is obtained from electrolysis of acidified water or obtained from cracking of alkanes.
- Glass beads increase surface area for the dissolving of hydrogen chloride gas. Glass beads control the absorption of HCl gas so that it can dissolve in water.
- Cl2(g) + H2 → 2HCl(g)
- X - Hydrogen gas
Question 3
Give the names of the following compounds.
- CH3 CH2 CH2 CH2 OH
- CH3COOCH2CH3
Answer
- Butan-1-ol
- Ethyletharioate
Question 4
One of the two formulae in the above question represents a sweet smelling compound. Give the names of the two organic compounds that can be used to prepare this compound in the laboratory.
Answer
Ethanoic acid and Ethanol.
Question 5
Ethane and Ethene react with Bromine according to the following equations given below:
- C2H6(s) + Br2(g) → C2H5Br(l) + HBr(g)
- C2H4 + Br2(g) → C2H4Br2(l)
Answer
- Substitution
- Addition
