Question 1
Consider the diagram below
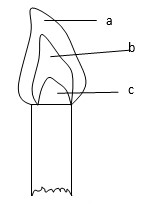
Name the regions labeled a, b, c.
- a
- b
- c
Answer
- a - Pale blue zone
- b - Green blue zone
- c - Almost colorless zone
Question 2
Some moist iron wool was placed in a test tube and the tube was inverted and set up as shown below.
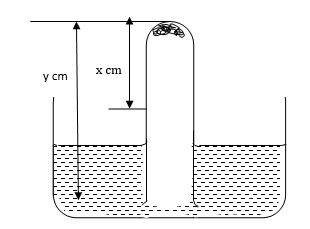
The apparatus was left for one week. The water level rose and iron wool turned red-brown.
- Write the chemical equation to show the rusting of iron.
- Write the expression for an approximate percentage.
- State two similarities between rusting and combustion.
Answer
- 4Fe(s) + 302(g) + xH2O → 2Fe2O3•xH2O(s)
-
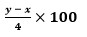
- 1. Both use oxygen
2. Oxides are formed
Question 3
Paper chromatography is a method of separating colours or dyes.
What two properties should the components of a mixture have that would make the separation possible.
Answer
• Solubility in the solvents
• Stickiness or absorbility
• Stickiness or absorbility
Question 4
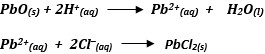
A student was asked to prepare Lead (II) chloride salt using the following ingredients; Nitric acid, lead (II)oxide and hydrochloric acid. Using ionic equations only explain how the salt can be prepared.
Answer
Question 5
The nitrates of the following metals were heated strongly and observation made accordingly. The nitrate of metal P produced the metallic oxide, Nitrogen (IV) oxide and oxygen gas; and that of metal Q produced the metallic nitrite and oxygen gas. The nitrate of R produced metal R, Nitrogen (IV) oxide and oxygen gas. Arrange the metals in order of reactivity beginning with the most reactive.
Answer
Q, P, R
