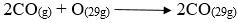Question 1
The set up below was used to separate a mixture of two substances.
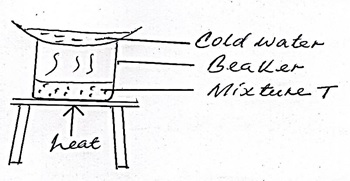
One of the components in the mixture T was sodium chloride.
- Name the other component
- Name the method of separation
Answer
- Ammonium chloride/iodine/iron (ii) chloride.
- Sublimation
Question 2
Dry hydrogen gas was passed through a hot metal oxide as shown in the diagram below.
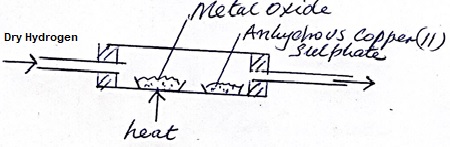
The metal oxide turned from black to brown.
- Identify the metal oxide
- State one observation in the combustion tube.
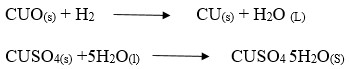
- Write a chemical equation for one of the reaction taking place in the combustion tube.
Answer
- Copper (ii) oxide
- Anhydrous copper (II) sulphate turn from white to blue.
-
Question 3
The diagram below shows regions of a burning charcoal stove.
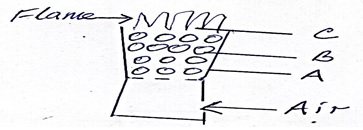
- Write an equation for the reaction taking place at
- B
- C
- Name the allotropes of carbon represented by the diagram below

Answer
-
-
- X - Graphite
Y - Diamond
Question 4
Student are advised to use a non- luminous flame when heating during laboratory experiments.
- Why is the non-luminous flame preferred.
- How does a Bunsen Burner produce a non-luminous flame.
Answer
- - It is hotter
- Does not form soot - By opening air holes fully.
Question 5
State Boyles Law.
Answer
The volume of a fixed mass of gas is inversely proportional to pressure at constant temperature.