Question 1
What is the meaning of the term homologous series?
Answer
Homologous series - a group of organic compounds that have similar chemical properties and structural features, with each successive member of the series differing from the previous one by a specific unit, such as a -CH2- group.
Question 2
Describe the procedure of preparing a soapless detergent from dodecene.
Answer
Dodecene is reacted with benzene to form alkylbenzene. The alkylbenzene is reacted with sulphuric (VI) acid to form alkylbenzene sulphonate. The alkylbenzene sulphonate is reacted with sodium hydroxide to form a soapless detergent.
Question 3
State three factors that affect the rate of a chemical reaction.
Answer
• Concentration
• Temperature
• Catalyst
• Light
• Surface area
• Temperature
• Catalyst
• Light
• Surface area
Question 4
In an industrial process, ammonia is produced by reacting nitrogen gas and hydrogen gas in the presence of a catalyst.
- State Le Chatelier's principle
- Write the equation for the formation of ammonia gas
- State how an increase in temperature affects the position of equilibrium
Answer
- Le Chatelier's principle - When a change is applied to a system at equilibrium, the system moves so as to oppose that change.
- N2(g) + 3H2(g) ⇌ 2NH3(g)
- The reaction is exothermic. An increase in temperature favours the endothermic reaction which is the reverse reaction. The equilibrium shifts to the left.
Question 5
The diagram below shows the relationship between the physical states of matter. Study it and answer the questions that follow.
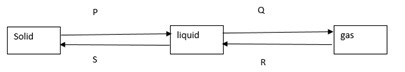
- Identify energy changes represented by the letters.
P
Q - Explain why there is no change in temperature during each of the processes shown in the diagram.
Answer
- P - Latent heat of fusion
Q - Latent heat of vaporisation - The heat energy is used to break or weaken the bonds during the processes and hence no changein temperature.
